Daedalus could learn from a team of physicists from Great Britain and Switzerland. Taking the principles of fractal geometry and the strategic game of chess, they created what they say is the most diabolical maze ever created.
The group, led by physicist Felix Flicker of the University of Bristol in the United Kingdom, created paths called Hamiltonian loops in patterns known as Ammann-Benker mosaics, creating complex fractal mazes that they say describe an exotic form of matter known as quasicrystals.
And all this was inspired by the movement of a knight around a chessboard.
“When we looked at the line patterns we created, we realized they made incredibly complex mazes. The subsequent mazes increase exponentially in size, and there are infinitely many of them,” Flicker explains.
“A chess piece that jumps two squares forward and one to the right on its knight’s turn visits each square of the chessboard only once before returning to its starting square. This is an example of a ‘Hamilton cycle’; a cycle that visits all stops on the map only once.”
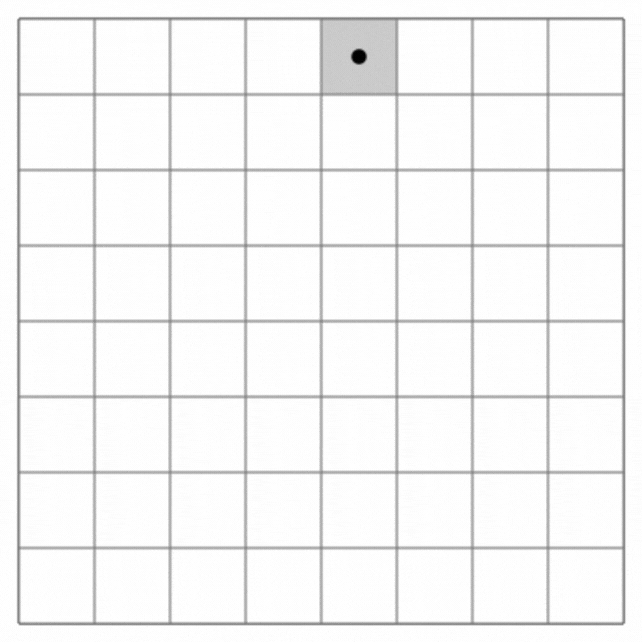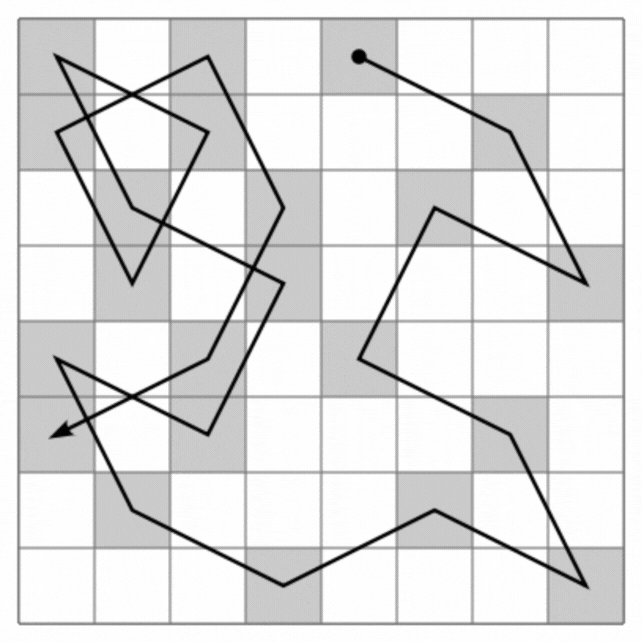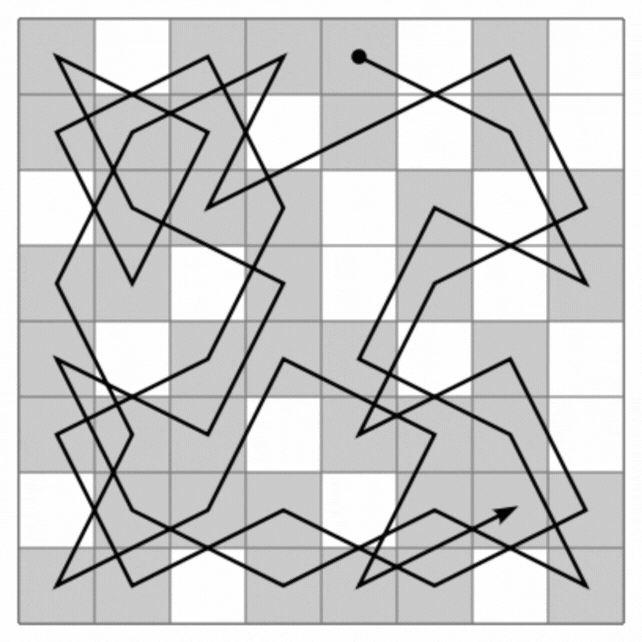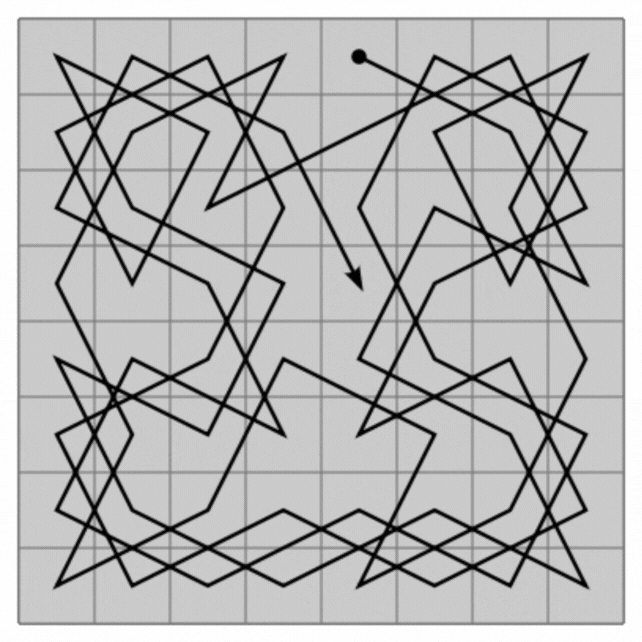
Quasicrystals are a very rare form of matter in nature, a strange hybrid of regular and irregular crystals in solids.
In a regular crystal (salt, diamond, or quartz), the atoms are arranged in a very precise pattern that repeats itself in three dimensions. You can take one part of this lattice and put it on top of another, and they match up perfectly. A disordered or amorphous solid is one in which all the atoms are odd. These include glass and some types of ice not normally found on Earth.
A quasicrystal is a material in which the atoms form a pattern, but this pattern does not repeat itself exactly. It may appear quite similar, but overlapping parts of the pattern will not match.
These patterns, which appear similar but are not identical, are very similar to a mathematical concept called aperiodic tessellations, which involve patterns of shapes that do not repeat in the same way. One of these is the famous Penrose tile. Ammann-Benker tiles are different.
Flicker and his colleagues, physicists Shobna Singh of Cardiff University in the United Kingdom and Jerome Lloyd of the University of Geneva in Switzerland, used a series of two-dimensional Ammann-Binker mosaics to create Hamiltonian loops that they say describe the structure of a quasicrystalline atom. . .
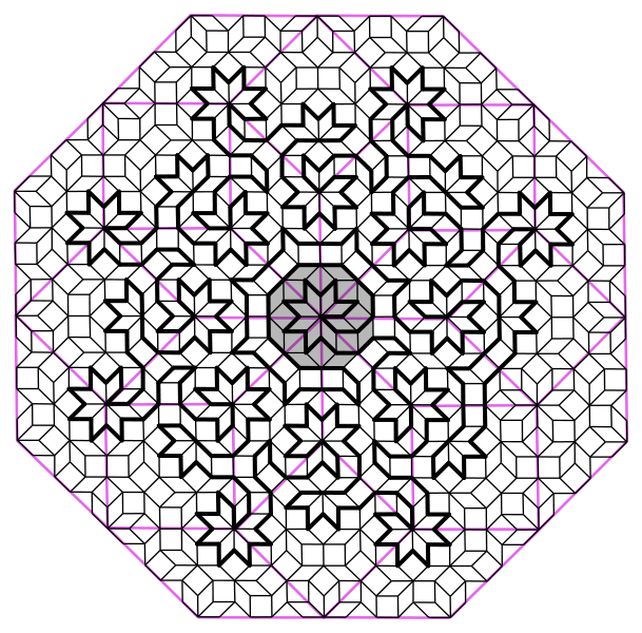
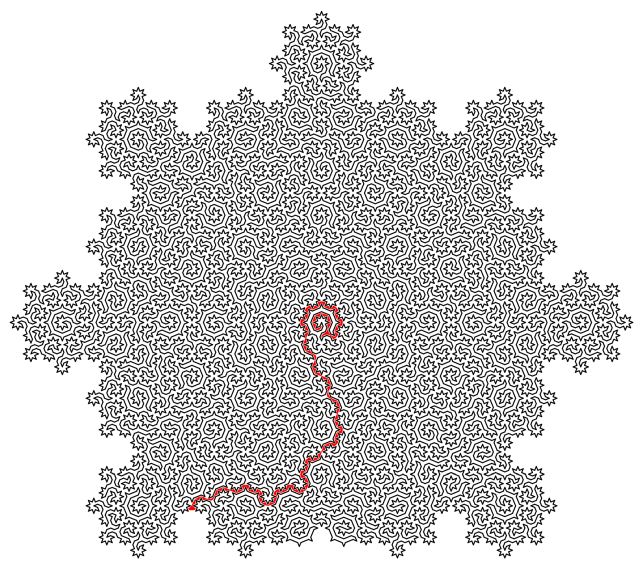
The loops they create visit each atom in the quasi-crystal only once, connecting all the atoms in a single line that never intersects itself but clearly continues from beginning to end. And this can scale infinitely, creating a type of mathematical pattern known as a fractal, in which the smallest parts resemble the largest.
This line then forms a maze with a starting point and an exit. But the research has much bigger implications than entertaining cranky kids at restaurants. On the one hand, Hamiltonian cycles are incredibly hard to find. A solution to describe Hamiltonians has the potential to solve many other complex mathematical problems, from complex pathfinding systems to protein folding.
And interestingly, there are implications for carbon capture via adsorption, an industrial process that involves molecules being transferred into a liquid by sticking them to crystals. If we could use quasicrystals for this process instead, the flexible molecules could be packed more tightly and stretched along the Hamiltonian loop inside them.
“Our work also shows that quasicrystals may be superior to crystals for some adsorption applications,” Singh says.
“For example, flexible molecules will find more ways to land on the irregularly spaced atoms of quasicrystals. Quasicrystals are also brittle, meaning they break up easily into smaller grains. This maximizes their surface area for adsorption.” And if you have a minotaur you need a place to hide, we think we know someone who can help. The study was published on Physical Examination X.
Source: Port Altele
