Chemists have been working on synthesizing valuable materials from waste molecules for years. An international collaboration of scientists is exploring ways to use electricity to simplify the process. In their research published in the journal Nature CatalysisResearchers have shown that carbon dioxide, a greenhouse gas, can be converted very efficiently into a type of liquid fuel called methanol.
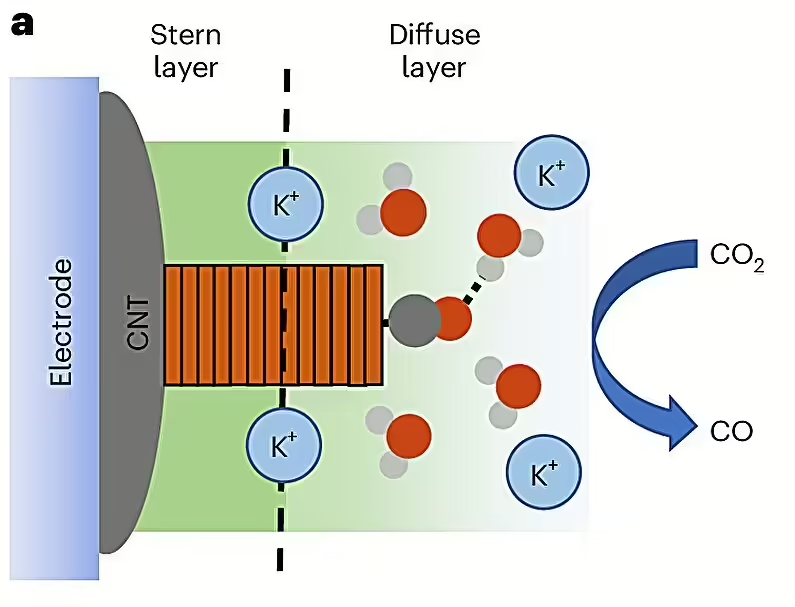
This process was accomplished by taking molecules of cobalt phthalocyanine (CoPc) and evenly distributing them over carbon nanotubes, graphene-like tubes with unique electrical properties. They had an electrolyte solution on their surface that allowed the CoPc molecules to accept electrons by passing an electric current through them and use them to convert carbon dioxide into methanol.
Using a special technique that relies on in situ spectroscopy to visualize the chemical reaction, the researchers saw for the first time how these molecules are converted into methanol, or carbon monoxide, which is not the desired product. They found that the reaction pathway depends on the environment in which the carbon dioxide molecule reacts.
Tuning this environment by controlling the distribution of the CoPc catalyst on the surface of the carbon nanotubes allowed for an eightfold increase in the probability of methanol formation from carbon dioxide. The discovery could improve the efficiency of other catalytic processes and have broad implications for other fields, said Robert Baker, co-author of the study and professor of chemistry and biochemistry at The Ohio State University.
“When you take carbon dioxide and convert it into another product, you can create a lot of different molecules,” he said. “Methanol is definitely one of the most desirable because it has a very high energy density and can be used directly as an alternative fuel.”
While the conversion of waste molecules into useful products is not a new phenomenon, until now researchers have mostly been unable to observe how the reaction actually happens – a crucial insight into the possibility of optimizing and improving the process.
“We can empirically optimize how something works, but we don’t really have a good understanding of what makes it work or what makes one catalyst better than another,” said Baker, an expert in surface chemistry, which studies how chemical reactions change over time as they occur on the faces of different objects. “That’s a very difficult question to answer.”
But with the help of specialized techniques and computer simulations, the team has gotten much closer to understanding the complex process. In this study, the researchers used a new type of vibrational spectroscopy that allows them to see how molecules behave on the surface, and its sophisticated measurements were crucial to the discovery, said Kwansong Zhu, lead author of the study and former Ohio State Presidential Scholar.
“We could tell from the vibration signatures that this was the same molecule in two different reaction environments,” Zhu said. “We were able to correlate that one of these reaction environments was responsible for the production of methanol, a valuable liquid fuel.”
A deeper analysis also revealed that these molecules interact directly with supercharged particles called cations, which enhance the methanol formation process, according to the study.
More research is needed to learn more about what else these cations enable, Baker said, but the discovery is key to a more efficient way to produce methanol.
“We are seeing very important systems and learning things about them that we have long wondered about,” Baker said. “Understanding the unique chemistry occurring at the molecular level is critical to taking advantage of these applications.”
Methanol produced from renewable electricity could be a low-cost fuel for vehicles such as aircraft, cars and boats, as well as be used for heating and power generation, as well as to advance future chemical discoveries.
“There are a lot of exciting things that could come next based on what we’ve learned here, and we’re already starting to do some of those things together,” Baker said. “The work is ongoing.”
Source: Port Altele