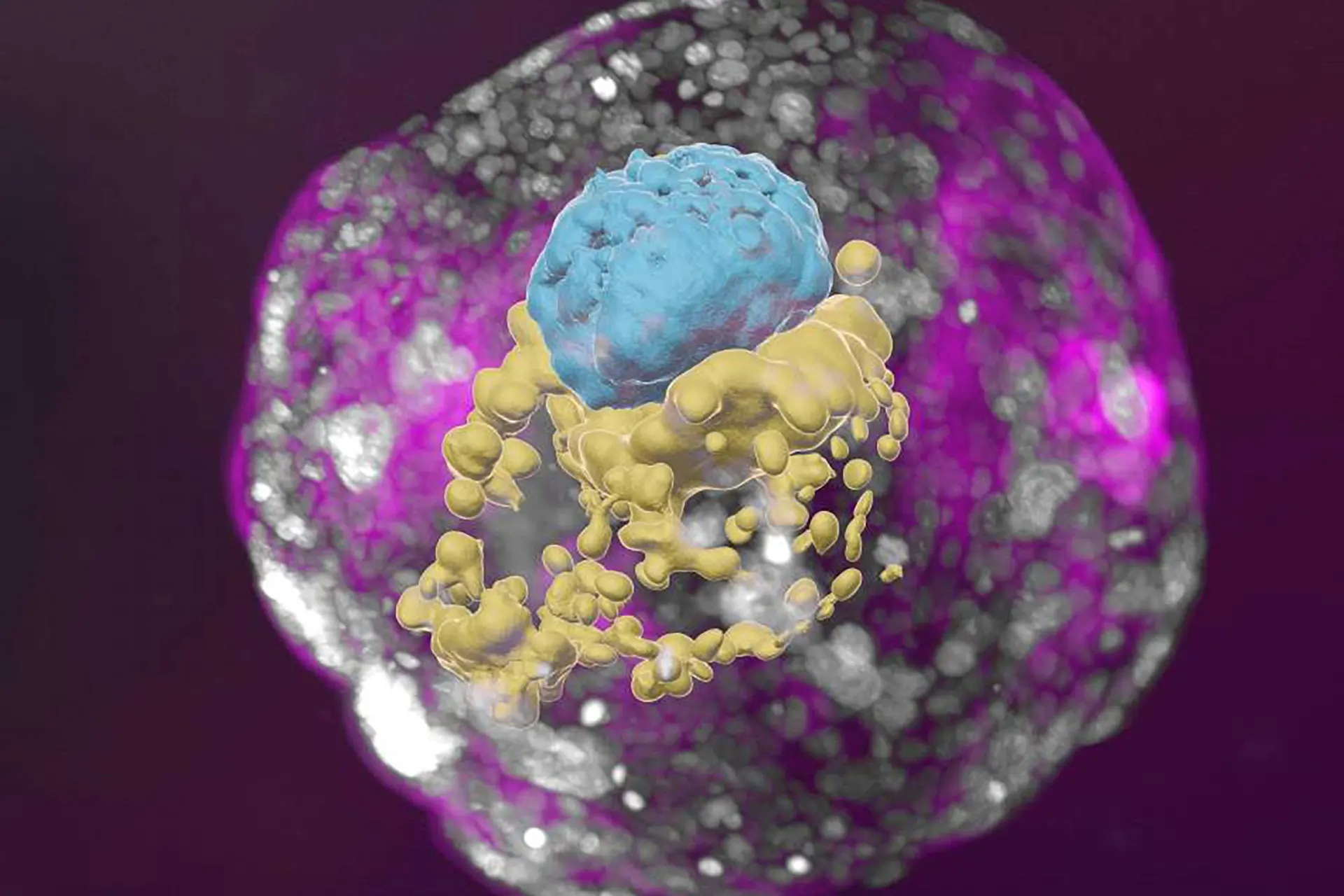
Researchers used pure pluripotent stem cells to create a model embryo that looks and moves like a natural human embryo. They say this is an ethical way to better understand embryonic development, which could lead to new avenues of research on birth defects and infertility.
Between 8 and 10 days after fertilization, the egg passes through the fallopian tubes and penetrates the uterine wall, marking the beginning of pregnancy (in medical terms). After implantation, the embryo continues to grow and develop. Major organs and systems of the body are formed during this period, making the developing embryo vulnerable to birth defects.
Working to better understand the complex development of embryos is difficult for ethical and technical reasons. But researchers at the Weizmann Institute of Science in Israel may have found a way to do just that.
“Drama in the first month; “The remaining eight months of pregnancy consist mostly of intense growth,” said study author Jacob Hanna. “But that first month is still mostly a black box. Our stem cell-derived human embryo model suggests that looking inside that box is ethical and cost-effective.” “It provides a way to accurately simulate the development of a real human embryo, including the appearance of its exquisite architecture.”
The researchers drew on their previous experience creating synthetic models of mouse embryos made entirely from stem cells. As in previous studies, they started with pluripotent stem cells that have the ability to differentiate into many, but not all, cell types. But researchers reprogrammed pluripotent stem cells to revert to an even earlier state, known as the naïve state, making them capable of differentiating into any cell type.
They divided pure pluripotent stem cells into three groups. Those that would turn into embryos were left as they were. The cells in the other two groups were treated only with chemicals, meaning they were not genetically modified to turn on specific genes designed to enable them to differentiate into one of the three types of tissue needed to support the embryo. Shortly after mixing, the cells coalesced and approximately 1% of them self-organized into full embryo-like structures.
“An embryo is, by definition, self-governing; We don’t need to tell him what to do; we just need to unlock its internally encoded potential,” said Hannah. “It is critical to mix the right cell types, which can initially only be derived from developmentally unrestricted naïve stem cells. When you do this, the embryo-like model itself will say: “Go!”.
The embryo-like structures developed normally outside the womb for eight days, reaching a developmental stage equivalent to day 14 of human embryonic development, the time when natural embryos acquire the structures that allow them to begin developing body organs.
The researchers found that the model embryos were structurally similar to the natural human embryos seen in old textbooks. They even observed the presence and activity of cells that produce human chorionic gonadotropin (hCG), the hormone used in pregnancy testing. Using extracts from these cells for home pregnancy testing gave positive results.
“Many miscarriages occur in the first few weeks, often before a woman even knows she is pregnant,” Hannah said. “There are also many birth defects, although they appear much later. “Our models can be used to identify the biochemical and mechanical signals that ensure proper development at this early stage and how this development can go wrong.”
Their work has already opened a new direction for future research. The researchers found that if an embryo is not properly enveloped by the cells that make up the placenta by day three, which corresponds to day 10 of natural embryonic development, its internal structures do not develop properly.
“An embryo is not static,” Hannah said. “It must have the right cells in the right organization and be able to progress; It’s about existence and becoming. “Our complete embryo model will help researchers answer fundamental questions about what determines proper growth of the embryo.”
Embryo models could reveal the cause of birth defects and types of infertility, and lead to new technologies for growing tissues and organs for transplantation, researchers say. It could also offer a way to conduct experiments, such as determining the effects of drugs on development, without involving live embryos. Source
Source: Port Altele