Scientists from the Paul Scherrer Institute and ETH Zurich have discovered a mechanism by which proteins form tiny liquid droplets that act as a smart glue in cells. These droplets adhere to the ends of the microtubules, helping to position the cell nucleus correctly during division. a published study Nature Cell Biologyexplains the age-old mystery of how the mobile protein structures in cells fit together.
The connections between the moving parts of the machines are very important for their smooth operation. Whether rigid or flexible, such as the connections between shafts in a motor or joints in a housing, the properties of the material ensure the correct transmission of mechanical forces. This is especially true for cells where interactions between mobile subcellular structures are essential for many biological processes. However, how nature provided this connection has puzzled scientists for a long time.
Now, studying the bond important for yeast cell division, researchers have discovered that for this, the proteins interact to condense into a drop of liquid. The work was the result of collaboration between the teams of Michel Steinmetz from the Paul Scherrer Institute PSI and Yves Barral from ETH Zurich, with the help of the groups Eric Dufresne and Jörg Stelling at ETH Zurich.
Proteins achieve ideal material properties to provide biological function by forming a liquid droplet. According to Barral, whose research group studies the process of cell division in yeast, this discovery is just the beginning of a new understanding of the role of smart fluids in the cell. “We are finding that liquids made up of biomolecules can be extremely complex and exhibit a much wider range of properties than we are used to from our macroscopic perspective. In that regard, I think we will find that these liquids have incredible properties that have been chosen by evolution over 100 million years.”
Microtubules: cell junctions
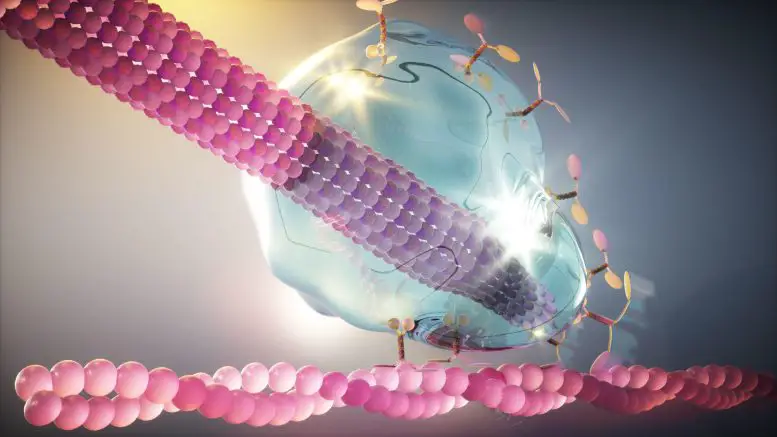
The research focuses on the connection that occurs at the ends of microtubules, filaments that cross the cell’s cytoplasm and bear a disturbing resemblance to alien tentacles. Created from the building block of tubulin, these hollow tubes act as traction ropes, carrying various cargoes throughout the cell.
Microtubules receive one of the most important loads during cell division. In yeast, they perform the important function of dragging the nucleus containing the dividing chromosomes between parent and budding daughter cells. For this, the microtubule must be attached to the actin cable, which is fixed to the cell membrane of the new daughter cell, with the help of a motor protein. The motor protein then moves along the actin cord, pulling the microtubule into the daughter cell until the cargo of valuable genetic material reaches its destination between the two cells.
This connection, necessary for further cell division, must withstand tension during the movement of the motor protein and allow the nucleus to maneuver with precision. Michel Steinmetz, a research group at PSI who specializes in the structural biology of microtubules, explains: “There has to be an adhesive between the microtubules and the motor protein. Without it, if the microtubule splits, you end up with a daughter cell with no genetic material that would not survive.”
Nature’s flexible connection
In yeast, three proteins that make up the core of the so-called Kar9 network adorn the tip of the microtubule to provide this connection. How they achieved their desired material properties seemed to contradict the traditional understanding of protein interactions.
A question that has long puzzled scientists is how the three core Kar9 network proteins remain attached to the microtubule tip even when tubulin subunits are added or removed: the equivalent of having the hook at the end of a tow rope stay in place with adjacent segments of the strand. is inserted. or cut. Here’s their discovery provides an answer: Just as a drop of liquid glue sticks to the tip of a pencil, this protein can adhere to the tip of a “liquid” microtubule even as it grows or shrinks.
To achieve this fluid property, the researchers found that the three core proteins of the Kar9 network cooperate through a network of weak interactions. Because proteins interact at several different points, if one interaction fails, others remain and the “glue” is mostly preserved. Researchers believe this gives the microtubule the flexibility it needs to remain attached to the motor protein, even under stress.
To make their discovery, the researchers methodically investigated the interaction between the three protein components of the Kar9 network. Based on the structural information obtained in previous studies at the Swiss Light Source SLS, they can mutate proteins to selectively remove interaction sites and observe effects in vivo and in vitro. Source
Source: Port Altele