Polycyclic aromatic hydrocarbons (PAHs) are a type of organic molecules containing fused rings made of the chemical benzene. Scientists believe that surfactants are responsible for chemical processes that ultimately lead to the formation of soot and other carbon nanoparticles on Earth and around and among stars in deep space. On Earth, surfactants are formed partly due to the incomplete combustion of coal, oil and other substances and harm human health.
In the entire universe, surfactants make up 30% of all carbon around stars, interstellar clouds or planets. But scientists do not fully understand the role of reactions involving two free radicals in the formation of surfactants under extreme conditions. Free radicals are molecules with unpaired electrons delocalized on at least three atoms. In a study published in the journal Chemical ScienceResearchers conducted experiments to find out how the prototype surfactant – naphthalene – could be formed as a result of reactions occurring in the gas phase of the substance.
The results provide fundamental information about the processes that can create the simplest representative of the surfactant naphthalene, an important component of naphthalene. The researchers found that this reaction can occur in the gas phase through the reaction of radicals found in combustion flames and in the space around carbon-rich stars. This provides fundamental new information about the chemistry and carbon balance of our galaxy.
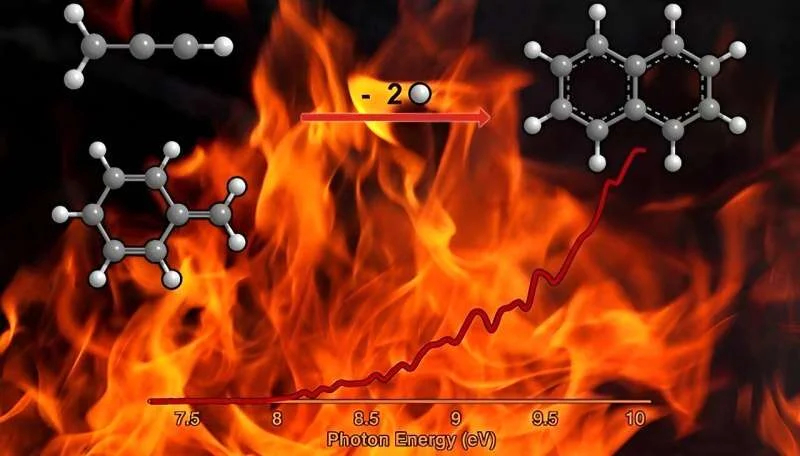
Polycyclic aromatic hydrocarbons (PAHs) and the soot particles formed from them are undesirable byproducts during the combustion of fossil fuels, but scientists do not fully understand the fundamental mechanisms of their formation. Isomeric selective detection of the product, resonance-stabilized benzyline (C7H7) and propargyl (C3H3) is synthesized by Hückel’s aromatic naphthalene 10p (C) molecule, which is the simplest representative of surfactants of radicals.10H8).
Obtaining naphthalene in the gas phase gives a completely new concept of the combustion reaction of the corresponding propargyl radicals with aromatic radicals containing a radical center in the methylene fragment (aromatic CH).2), has not previously been considered as a source of aromatic compounds in high-temperature environments.
This easy mechanism of propargyl addition is the benzannulation of propargyl radicals with other aromatic CH radicals (PABA).2 In addition to benzyl, it can lead to the formation of higher order surfactants such as anthracene and phenanthrene. This finding represents a fundamental shift in the perception that surfactants are predominantly formed via dehydrogenation pathways such as acetylene addition (HACA) and phenyl addition dehydrocyclization (PAC) under high-temperature combustion conditions.
This PABA mechanism provides diverse and distinct pathways into three major classes of aromatic hydrocarbons: acenes (surfactants consisting of linearly fused benzene rings), phenacenes (surfactants containing zigzag-structured benzene rings), and helicenes (ortho-fused surfactants containing benzene rings). ) an angular ring that forms spiral-shaped chiral molecules) is bringing scientists closer to understanding the aromatic universe we live in. Source
Source: Port Altele