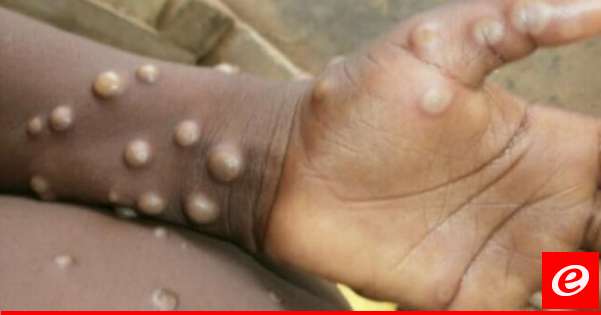
The U.S. Food and Drug Administration has approved a new monkeypox drug as cases of the rare virus have been found in 11 countries, including the United States and the United Kingdom. received the green light to administer intravenously a combination of TPOXX, a smallpox drug already available for oral administration.
The company notes that in Europe the drug has been officially approved for the treatment of monkeypox, as many smallpox drugs are caused by similarities between viruses.
The drug has been added to doctors as a rare but potentially devastating virus as it continues to spread around the world after it was confirmed or suspected in dozens of cases in 11 countries in Europe and North America.
TPOXX has been approved for use in Europe, the United States and Canada, the three places where monkeypox is most active outside of Africa.
Source: El Nashra